Subtotal: 5.389,10€ (incl. VAT)
Three takes on tomorrow’s materials
Lights, muscles, action
Ritu Raman’s engineered muscle cells contract in response to light—and could lead to biologically based robots that adapt to their environment or repair themselves after a crash.
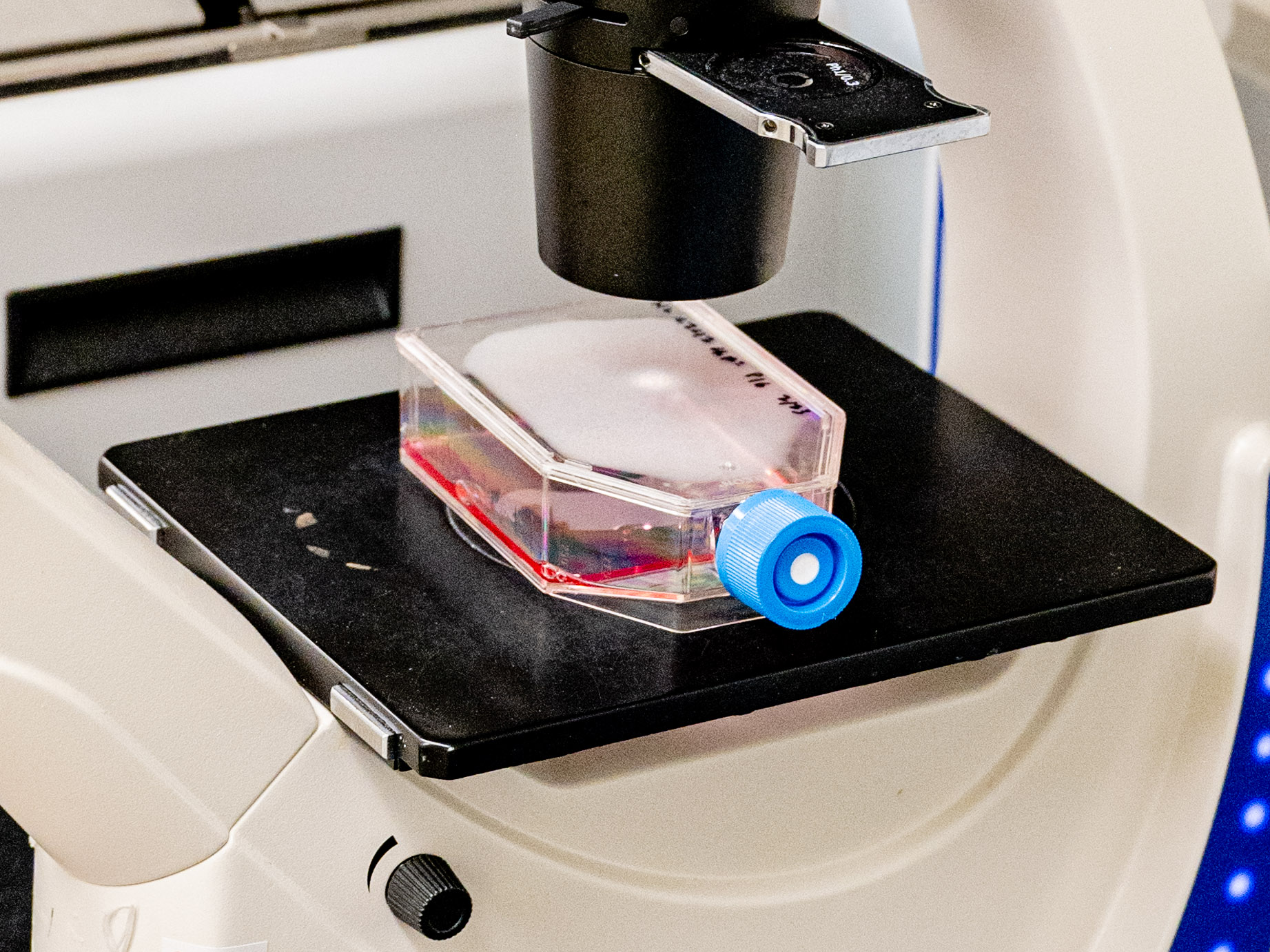
Ritu Raman rubs her gloved hands with ethanol and reaches into an incubator the size of a mini-fridge to pull out a tray of petri dishes. The dishes contain translucent, U-shaped 3D-printed scaffolds. And upon these polymer skeletons, small pink bands of muscle cells are growing.
She heads to the room next door, where a fiber-optic light can be positioned above the dishes, emitting pulses of blue light too bright to look at without safety glasses. Raman explains that the cells, which are from a line originally derived from mice, are bioengineered to contract under such a glow; the pulsing light acts like a personal trainer, causing them to exercise. “They live over there, and then they come here to go to the gym,” she jokes.

TOAN TRINH
In the past two decades, engineers have been experimenting with biological materials because biohybrid design holds a distinct advantage over building with plastic or steel: living cells can grow, change, and adapt. Raman, who is a Brit (1961) and Alex (1949) d’Arbeloff Career Development Assistant Professor in Mechanical Engineering, runs a lab focused on creating adaptive biological materials that take advantage of cells’ ability to sense, process, and respond to their environment.By measuring how light-induced activity affects her bioengineered cells, Raman can get a better idea of how biohybrid robots might one day be able to adapt to unfamiliar terrain.
“There are multiple things that can change when you exercise,” she says. Certain types of muscle fibers, for example, can only carry small loads, but they can do so for a long time; others can handle much higher force but tire easily. Which muscle fibers get stronger depends upon what kind of exercise the muscle performs. Raman envisions a biohybrid robot meant to operate remotely, powered by a “battery” of sugar and amino acids, that could be designed to develop the right muscles for the job at hand and even repair itself by regrowing parts damaged in a crash or fall.
“If I wanted a robot to go across the room, which is a relatively controlled environment with a constant temperature and everything else, I could just build a regular robot at low cost,” she says. “But in an unpredictable, dynamic environment, I might not know how strong it needs to be, or what dangers might be present. If it gets harmed, I won’t be able to go and heal it, so it needs to be able to recover and adapt.”
Raman grew up in India, Kenya, and the US, the daughter of a chemical engineer and a mechanical engineer. As her parents worked to solve real-world problems, she saw the immediate benefits that engineering could bring; she recalls watching her father install communication towers in rural villages. As a mechanical engineering major at Cornell, she randomly took a biomechanics course and was immediately hooked. “It was the first textbook I actually enjoyed reading in my entire life,” she says.
Not that biology was always fun. As a lab assistant, she worked in a lab that measured how alcohol and exercise together affected rats. “I was in a basement with drunk rats on treadmills that didn’t want to run,” she says. “It was terrible!” Gradually, however, she became fascinated by how the animals’ bodies and behavior changed in response to their unusual environment: “I was like, that one is so chunky, that one is strong, and that one has learned how to make the treadmill go so he doesn’t have to run. Nothing we can build matches how smart and adaptive living systems are.”
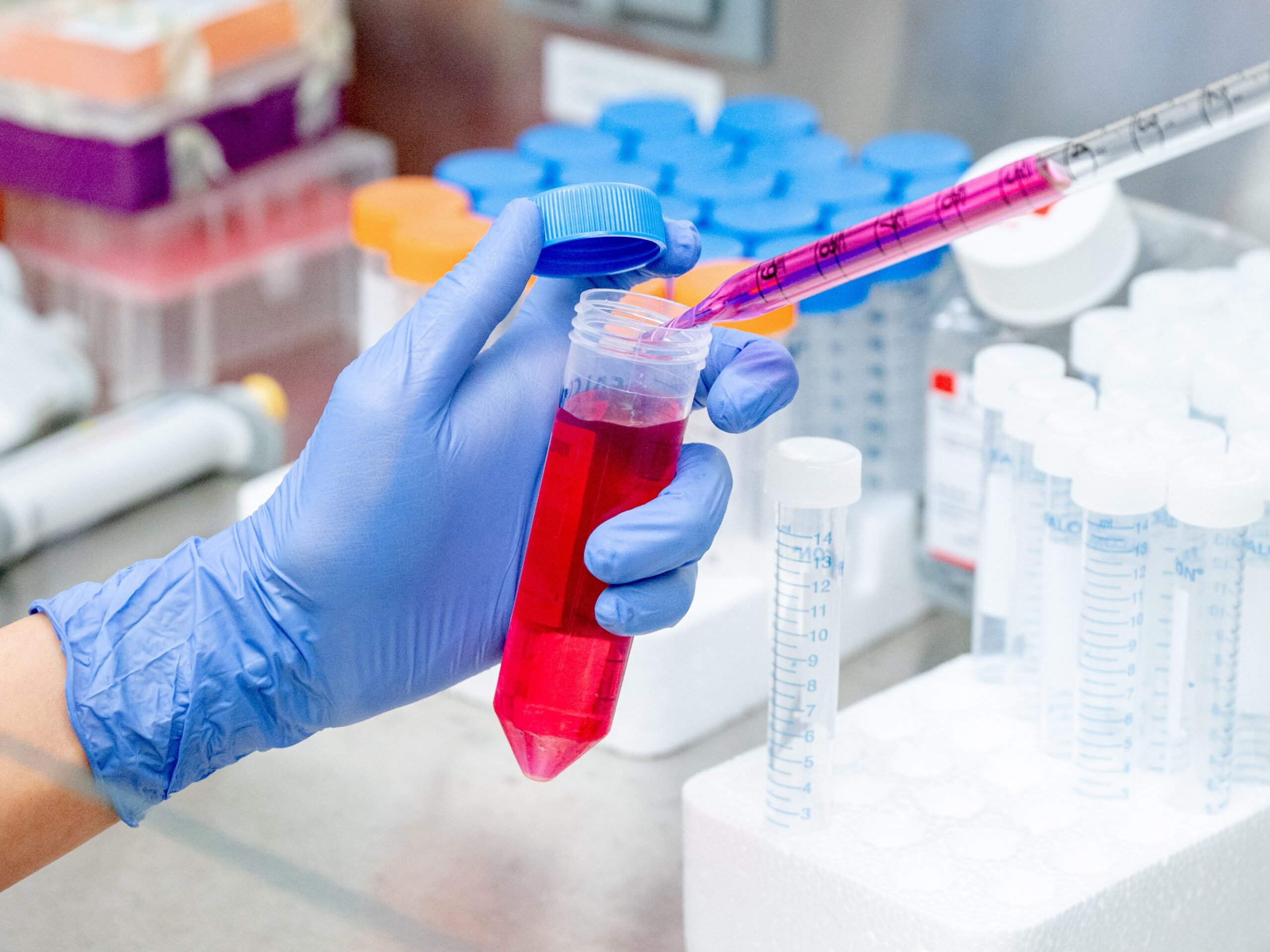
Raman transfers liquid cell-culture media (left) to a flask of living cells. Her engineered muscle cells are triggered by light flashes to contract or “exercise.”
Raman continued exploring how living systems adapt in her doctoral studies at the University of Illinois, where she participated in work funded by a large National Science Foundation grant to examine how muscles could grow and heal themselves. Normally, muscle cells contract in response to electrical signals sent by nerves through a voltage-gated ion channel in the cell membrane. Raman wanted to develop muscle cells that would contract in response to light instead. So she collaborated with Roger Kamm, SM ’73, PhD ’77, an MIT professor of biological and mechanical engineering, and used genetic engineering to insert into mouse muscle cells a light-gated ion channel that others had developed from green algae cells. She showed that by shining a light on the cells at regular intervals to induce them to contract and release repeatedly, she could make them get stronger and recover from damage.
In a postdoc at MIT with bioengineering pioneer Robert Langer, she demonstrated this in a living animal. After removing a chunk of muscle from a mouse’s leg, she implanted the engineered light-sensitive muscle at the injury site and stimulated it to exercise by shining light through the skin. “The mice completely recovered their mobility a week after the damage,” she says.
Since joining the MIT faculty in the fall of 2021, she has started working on optimizing the exercise regimens needed to develop muscles best suited for particular tasks—and figuring out how to control that process by manipulating such things as the brightness and timing of the light pulses. Her lab is also experimenting with other ways to control muscle cells, including integrating neurons into the tissues to mimic how they’re controlled in an organism.
Eventually, she’d be interested in developing soft, muscle-actuated robotic tools that would be more precise than the metal tools surgeons currently rely on. Larger robots made of living cells could operate in challenging environments and do things like crawl around a water filtration system to remove sources of contamination. “Imagine the robot could carry other cells that produce chemicals or proteins to neutralize a toxin,” Raman says. “So it’s not just about movement—it’s also about sensing and responding in other ways.”
“We’re not trying to replace the materials that engineers typically build with,” she says. Rather, she wants the next generation to think of living cells as something else they can use.
Painting with light
Stefanie Mueller imagines a world where you could change the color of your shoes as easily as you change a digital avatar.
When you’re in the market for a new car, you can often go online to virtually “paint” one different colors to see which you’d prefer. But what if you could do that in real life—and change the color of your car to suit your mood? Stefanie Mueller, the TIBCO Career Development
Associate Professor of EECS with a joint appointment in mechanical engineering, is working to make that possible. “The main vision for our lab,” says Mueller, “is to give physical objects digital capabilities.”
Mueller, who leads MIT’s Human-Computer Interaction Engineering Group, has pioneered a technique using light-activated inks, known as photochromic dyes, that could change the color of an object in mere minutes. Sitting on a black leather couch in her office in the Stata Center, she pulls up a video to demonstrate. A 3D-printed model of a chameleon sits within a glass case. When a projector shines a light on it, a white-and-brown zebra-striped pattern gives way to a multicolored checkerboard.

GRETCHEN ERTL
“This is the same object,” she says. “We spray our smart material onto it with an airbrush, and then once it’s on, you project some light onto it and can reprogram its appearance.” The color pattern doesn’t go away when the light stops shining; it stays for up to 26 hours, or until a new one is projected. Mueller’s emphasis is not so much on creating new materials—photochromic dyes have existed for decades—as on developing new processes and techniques to create dazzling new effects with existing ones.
This technique, which she calls Photo-Chromeleon, makes use of photochromic dyes in yellow, cyan, and magenta. “It’s similar to how your ink-jet printer works,” Mueller says. Photochromic dyes can be switched on (if exposed to UV light) or off (if exposed to light of a specific wavelength in the visible spectrum). The three dyes are mixed into a transparent lacquer that’s airbrushed onto an object. To create a multicolored design, all three dyes are activated with UV light, turning the object black. Then the dyes are selectively deactivated by shining light of specific wavelengths on the dyed surface. Since the wavelengths of red, green, and blue light deactivate cyan, magenta, and yellow, respectively, a standard office projector with red, green, and blue LEDs can be used to control all three dyes. For example, the red LED deactivates cyan, leaving magenta and yellow, which combine to create red. By projecting pixels of red, green, and blue light onto an object, Mueller can “paint” it with very high resolution. And adjusting the length of exposure to the different wavelengths makes it possible to achieve intermediate colors. The process could be used with a range of objects, including smartphone cases, shoes, and T-shirts.
Although currently the color fades within a few hours when exposed to sunlight, Mueller’s team is working on embedding tiny LEDs into a flexible substrate to create textiles that could preserve the designs by refreshing the photochromic particles.
Mueller envisions a time when we wouldn’t need to purchase new things to change our style. “In the future, maybe a shoe company will give you a shoe for free,” she says, “but you will get a subscription to an app, and download patterns”—patterns you could apply to the shoe. (Her lab is developing a portable reprogrammer that could be used anywhere to refresh or update designs.) Beyond allowing people to change their styles as easily as they change a digital avatar, the technology could also reduce waste.
“Right now, companies make money by updating a trend so you buy more stuff—because they can’t make money from the same item again,” Mueller says. This process, however, would flip their incentives, making it more lucrative not to manufacture something new. “They could make the new trend just be selling you a pattern to unlock. They don’t have to give you something new physically.”
Mueller, who grew up in Germany and studied computer science at the Hasso Plattner Institute in Potsdam, was working on her PhD in the early 2010s when cheap 3D printers hit the market. She became fascinated with the idea of hacking the machines to do things like print with different materials. That got her thinking about how the materials themselves could be changed to give physical objects new capabilities. MIT’s Computer Science & Artificial Intelligence Laboratory (CSAIL), which she joined in 2017, has been the perfect fit, given its interdisciplinary nature.




In Mueller’s “Photo-Chromeleon” technique, projected light can be used to control the appearance of an object coated with a mix of photochromic dyes. (PHOTOS COURTESY OF THE RESEARCHER)
To make her color-changing items, for example, “you need to develop the material, put that material onto objects, and create an algorithm that computes how long you have to shine the light on each pixel—so
you need these three components of hardware, materials, and algorithms,” she says. “If you miss one of them, it’s not going to work.”
Another technique Mueller and her grad students are prototyping uses birefringent film, which changes appearance depending on the polarization of light. “It sounds really fancy, but it’s basically just like a food wrap,” she says. By layering this film in specific patterns and then applying a polarizing filter similar to the ones on sunglasses, her group can create objects that change their appearance when a dial is turned. For example, a map or anatomical model can take on different colors for teaching purposes.
Still other projects include developing user interfaces that can be sprayed onto surfaces. By layering conductive metallic ink, a dielectric, a phosphor, copper, and a clear conductor, they create sensors and displays such as a dimmer switch people can operate by running a hand along the wall. “They could use it as a slider to set the color or the brightness,” Mueller says. A transparent conductive material applied to the cushions of a couch can detect when someone sits on it. In one project, that triggers a photo album to open on a nearby screen, which is scrolled by swiping a hand above transparent electrodes sprayed onto the arm of the couch.
Like most of the projects Mueller chooses to pursue, these bring the things you can do on a computer into the physical realm. But speaking more generally, she is always looking for a “wow” factor. “If you look at a project and are like, ‘Wow, I can really see how it changes the world,’ then even if you don’t understand it, you want to know more about it,” she says. “We try and select ideas that have a big vision behind them, that will first draw in people so they can enjoy it. Then we can talk about all the science and technical details.”
Nano designs with a macro vision
Materials precisely designed at the nanoscale could have exciting applications—if they’re scaled up enough to make useful objects. Carlos Portela is developing new materials and techniques to make them at the macroscale.
If you drop a ceramic mug on the floor, chances are good that it will break. When the same ceramic material is extremely thin, however, something strange occurs, as Carlos Portela can demonstrate with a video. On his screen is a cube just 120 micrometers per side—eggshells are thick by comparison—made of a network of interconnected ceramic shells. Portela, a Brit (1961) and Alex (1949) d’Arbeloff Career Development Assistant Professor in Mechanical Engineering, points to one of the shell walls. “This is just 11 nanometers thick,” he says. That’s equivalent to about 30 atoms wide. “I’m going to compress [the cube] to half its height,” he adds. “What would you expect the ceramic to do?”
Any reasonable person would expect it to shatter into a hundred pieces. But when a load compresses the cube, it buckles and wrinkles like a sponge; when the load is removed, the cube springs back into shape. “This is basically the same material as a coffee mug,” says Portela with a grin, gesturing to one on his desk. “And remarkably, we don’t even see any cracks.” It’s like an entirely new substance.

TOAN TRINH
In all of human history, the materials we’ve built with—rock, metal, ceramic, plastic, and foam—have had a relatively limited range of physical characteristics, Portela says. To get one desirable property, builders often must compromise on another. Hard materials aren’t very light, for instance, and light materials aren’t very stiff.
In the last decade, however, engineers have begun designing at the nanoscale to create new materials that combine desirable properties never previously found together. Known as architected materials or metamaterials, they are combinations of materials with well-known properties, such as ceramics and polymers. But manipulating how they’re constructed at the nanoscale makes them behave completely differently from their familiar precursors. Portela says that carbon structures could be both strong and energy-absorbing, and metallic materials could be engineered to be superlight. Other materials could be made to act as lenses that can focus acoustic waves. Given that the biggest limiting factor for airplanes and rockets is the weight of the materials they are built with, new materials that are both strong and lightweight could dramatically increase the distance they can fly on a given amount of fuel.
A silver airplane model in Portela’s window overlooking Killian Court attests to his early love for airplanes. Growing up in Colombia, he wanted to become a pilot. He studied aerospace engineering at the University of Southern California and got his pilot’s license, but as an international student, he had difficulty securing an internship at a major aircraft company. By then, he had become fascinated by the potential for nanoengineering and entered a PhD program in the topic at Caltech, where he studied with Julia Greer ’97, a pioneer in architected materials. Greer was experimenting with using finely calibrated 3D printers to create intricate nanoscale lattices that could become materials with new properties. “Her energy and passion for this was infectious,” Portela says. “It made me say, ‘I want to do this.’”
As revolutionary as the techniques are, however, they are also limited. A printer can take weeks, if not months, to print a cube just a few millimeters thick, making it tedious to design and create new objects. “Real-life applications require you to make a nanomaterial large enough to hold in your hands,” Portela says. That’s where his research comes in. He has been developing new techniques for making architected materials, some of which don’t involve a 3D printer at all.
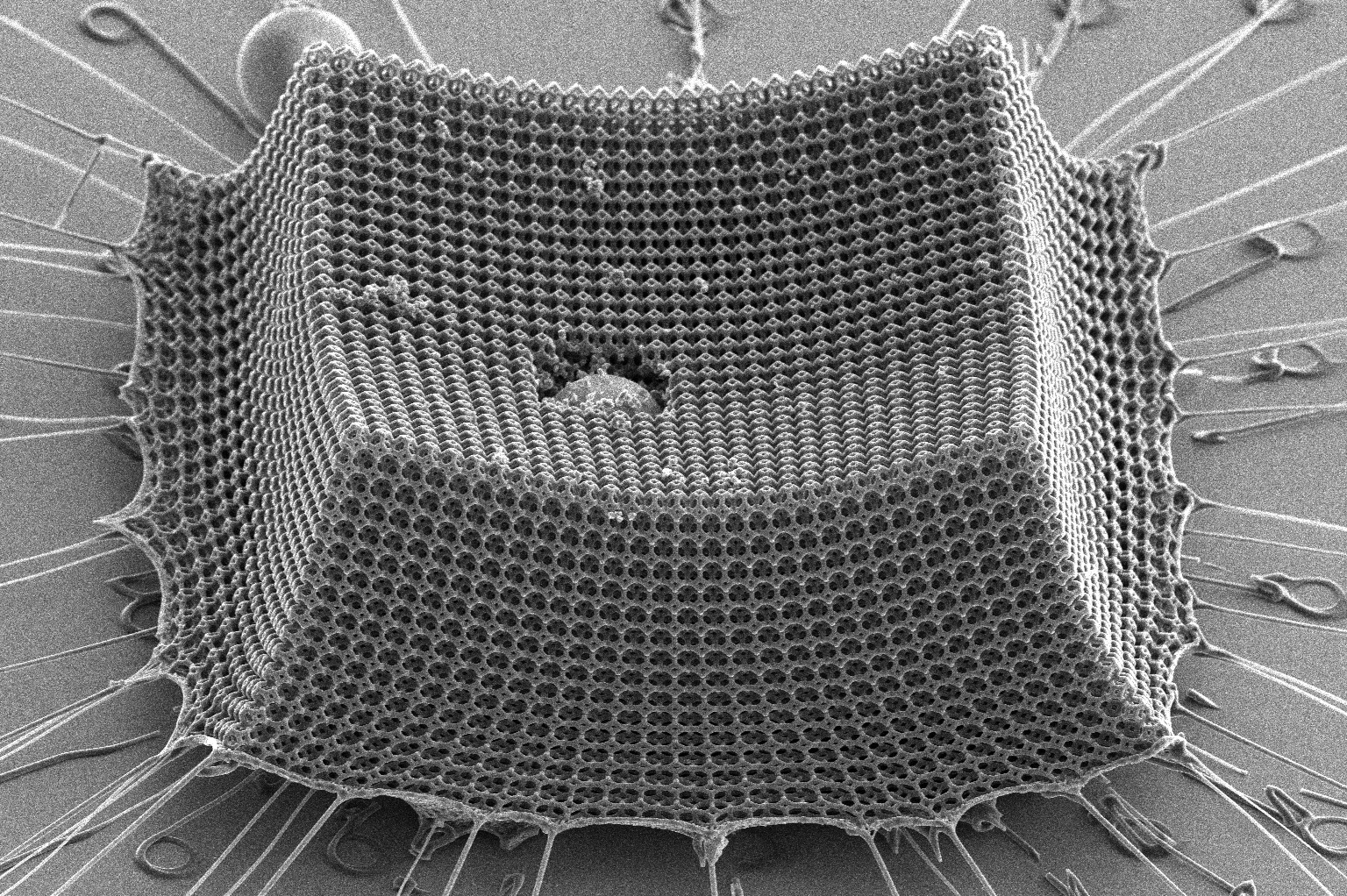
COURTESY OF THE RESEARCHER
In one technique, he taps into the natural properties of the materials themselves by mixing together two polymers to form an emulsion, similar to shaking oil and water together. As heat is applied to make the two polymers begin to separate again, they naturally form an interlaced pattern on a microscopic scale. By solidifying them at that moment, and then using water to remove one of the polymers, he can create an irregular network that is just as intricate—and, surprisingly, just as strong—as a precisely printed lattice. “We can do this in a matter of hours, not months,” Portela says. Then he coats his structure with an ultrathin film of ceramic using atomic layer deposition, which involves alternately exposing it to vapors of two different chemical reactants. The remaining polymer is then typically removed from the ceramic-coated structure by exposing it to oxygen plasma, which causes it to decompose, leaving just the strong, porous ceramic shell. Portela says his centimeter-scale samples created with this technique are some of the largest self-assembled 3D nanomaterials ever made.
To create strong but light carbon material, Portela 3D-prints a polymer network, which then undergoes pyrolysis: it’s heated at temperatures upwards of 1,500 °C in an inert atmosphere, which burns off virtually everything but its carbon atoms. This results in a mass loss of about 90% and at least a tenfold increase in stiffness, creating strong but porous new materials. Portela has also created samples of micro-architected printed and pyrolyzed carbon materials at the cubic-centimeter scale. And he is working on even larger objects.
Portela’s group constructs some of their architected materials using a nanoscale-resolution 3D printer housed in MIT.nano, a shiny 100,000-square-foot facility completed in 2018. And they rely on MIT.nano’s equipment for atomic layer deposition and use its electron microscopes to capture video as they test their materials. The self-assembled materials, on the other hand, are made at Portela’s own lab in Building 31, where he and his grad students do additional mechanical testing and run computer simulations to predict the properties of the materials they’re creating. They also use the laser confocal microscopes at MIT’s Institute for Soldier Nanotechnologies for impact testing. In one experiment, his group fired supersonic particles at a carbon-steel lattice, showing that it was 70% more effective than Kevlar in stopping impacts. That opens up possibilities for an ultralight new form of body armor.
Portela is also collaborating with colleague Ritu Raman, whose office is next door to his, on architected materials that integrate biological components. They hope one day to develop materials that could more closely mimic the physical properties of human skin and tissue, which need to be both pliable and strong. Meanwhile, Portela is in the early stages of creating lightweight materials with potential applications like aircraft construction, as well as metamaterials that could be used to create ultraefficient filtration systems and more effective ultrasound devices.
His group is tackling questions that no one has tried to answer before because, he says, “they haven’t had the right experimental means to do this.”














