Subtotal: 31,94€ (incl. VAT)
This family raised millions to get experimental gene therapy for their children
When Gary Landsman prays, he imagines he is in Israel and his sons Benny and Josh are running toward him. They are wearing yarmulkes, and the cotton fringes called tzizit fly out from their waistbands. He opens his arms ready for a tackle.
The reality is Benny and Josh both have Canavan disease, a fatal inherited brain disorder. They are buckled into wheelchairs, don’t speak, and can’t control their limbs.
On Thursday, April 8, in Dayton, Ohio, Landsman and his family rolled the older boy, Benny, into a hospital where over several hours, neurosurgeons drilled bore holes into his skull and injected trillions of viral particles carrying the correct version of a gene his body is missing.
The procedure marked the climax of a four-year quest by the Landsman family, who live in Brooklyn, New York, to obtain a gene therapy they believe is the only hope to save their kids.
MIT Technology Review first profiled the Landsmans’ odyssey in the cover story of our 2018 special issue on precision medicine. Advances in gene therapy technology are making it possible to treat genetic diseases like hemophilia. But because Canavan is an ultra-rare disease, few companies are working on a cure. So the family financed the daring gene treatment on their own, using funds they raised online.
Impressive advances in genome sequencing, gene replacement, and gene editing mean, in theory, thousands of rare genetic diseases could be treated. But because companies aren’t leading the way, parents say, they are being forced to embark on multimillion-dollar quests to finance the needed experiments. Adding to the ethical dilemma: in some cases, parents are designating their own children as the first recipients.
The trial in Dayton, for instance, is prioritizing children whose families have been able to raise funds to underwrite the experiment, whose costs so far are close to $6 million. “It raises the eternal equity question of who gets access to trials and who doesn’t,” says Alison Bateman-House, a bioethicist at New York University who is studying ethical issues in pediatric gene-therapy trials.
The Landsman family has raised more than $2 million, and families from Russia, Poland, Slovakia, and Italy have also used cash donations to secure spots in the trial. A Russian family even posted a copy of an invoice for “gene-therapy treatment” in the amount of $1,140,000, which included $800,000 to offset costs of manufacturing the genetic treatment being used in the trial.
According to the Russian family’s urgent fundraising appeal, if they failed to pay that amount, their toddler Olga “will not receive the only chance for recovery—an expensive treatment in the United States.” They ended up contributing at least $700,000.
While such “pay-to-play” trials are legal, they do raise red flags, including questions about whether parents—and financial donors—understand that most experimental treatments fail. “They are not necessarily unethical. But you should scrutinize why the patient is being asked to pay,” says Bateman-House. “If it’s a valid trial, why isn’t the NIH [National Institutes of Health] interested, or a biotech company? Why isn’t there other funding?”
Will it work?
Canavan disease is caused when a child inherits two broken copies of a gene called ASPA. Without the enzyme that ASPA produces, the brain can’t correctly form the nerve bundles that transmit signals in the brain. The result, for Benny and Josh, is that the boys can’t speak or control their limbs, and their cognition is limited.
“They are like infants in ALS bodies,” says Paola Leone, the researcher at Rowan University in New Jersey who conceived the gene therapy and led the effort to get a clinical trial started.


The trial in Dayton seeks to use viruses to deliver working copies of the ASPA gene to kids’ brain tissue. That’s what occurred Thursday at the Dayton Children’s Hospital. After Benny was greeted by a golden retriever who cheers patients up, brain surgeons drilled into his skull and then used a needle to introduce 40 trillion virus particles.
Leone’s scientific bet is that adding correct copies of ASPA to specific brain cells called oligodendrocytes could stop the disease from progressing, and maybe allow for some recovery. The treatment has been effective in mice, she says, but “is that going to work in patients? The only way is to test it.”
Parents as scientists
There are by now a half-dozen examples of gene-therapy treatments funded by families aiming to treat their own kids, and more such experiments are planned. Scientists have even begun developing hyper-personalized medicine tailored to individual children who suffer from unique genetic problems.
These desperate efforts ask parents to overcome nearly impossible obstacles. They must become experts in drug development, raise millions, and tirelessly cajole scientists. Few people can pull it off.
“There are a lot of people who know how to do gene therapy, but the knowledge is all fragmented, and so much can go wrong,” says Sanath Kumar Ramesh, a software developer whose son is afflicted by a different rare disease. Ramesh founded an organization, Open Treatments, that is building software families can use to organize gene-therapy research, including steps such as hiring scientists to create animal models of an illness.
“I think in the future, the distinction between scientists and parents is going to be blurred,” he says.
For parents whose kids have already been accepted into the Dayton trial, gene therapy may be their last chance. One of them is Meagan Rockwell, a nail technician in Cedar Rapids, Iowa, whose daughter, Tobin Grace, now three and half, was diagnosed with Canavan in 2018.
“They told us sorry, there is nothing we can do—no treatment, no cure—you will be lucky if she sees her fifth birthday. It was a hard blow, to know your only child has a life-limiting brain disease,” Rockwell says.
Rockwell says she found out about Leone’s gene-therapy effort online and eventually raised more than $250,000. “At the time, Tobin was the youngest person in the US with Canavan, and I think that played a huge factor in her acceptance,” she says, adding that Leone tells parents money puts them at the front of the line but doesn’t guarantee treatment.
Bateman-House, the bioethicist, says another risk is whether parents can really judge the benefits of an experimental procedure in a “dispassionate” way, especially if they have sunk a fortune into the effort. “It’s not only that their child is facing a dangerous condition; it’s that their blood, sweat, and tears is what is funding this intervention,” she says. “It could be incredibly difficult for a parent to change their mind and say ‘We are not going to do this.’“
Hope versus risk
The Dayton study currently has enough supplies of the genetic drug to treat only nine or 10 children. It was manufactured in Spain, but only after the researchers and families overcame what they call an ordeal of red tape, delays, and obstacles, some thrown up by government regulators who decide which genetic treatments can be tried and whether trials are properly planned.
At one point, in 2019, the Landsmans took their sons to the US Food and Drug Administration for a meeting they landed after dozens of calls to lawmakers. “Beforehand we were a case number in their big pile of paper,” says Jennie Landsman, the boys’ mother. “They had very technical objections. In the meeting we held up Benny and Josh, and we said ‘We hope this issue that is so technical isn’t going to stop the treatment.’”
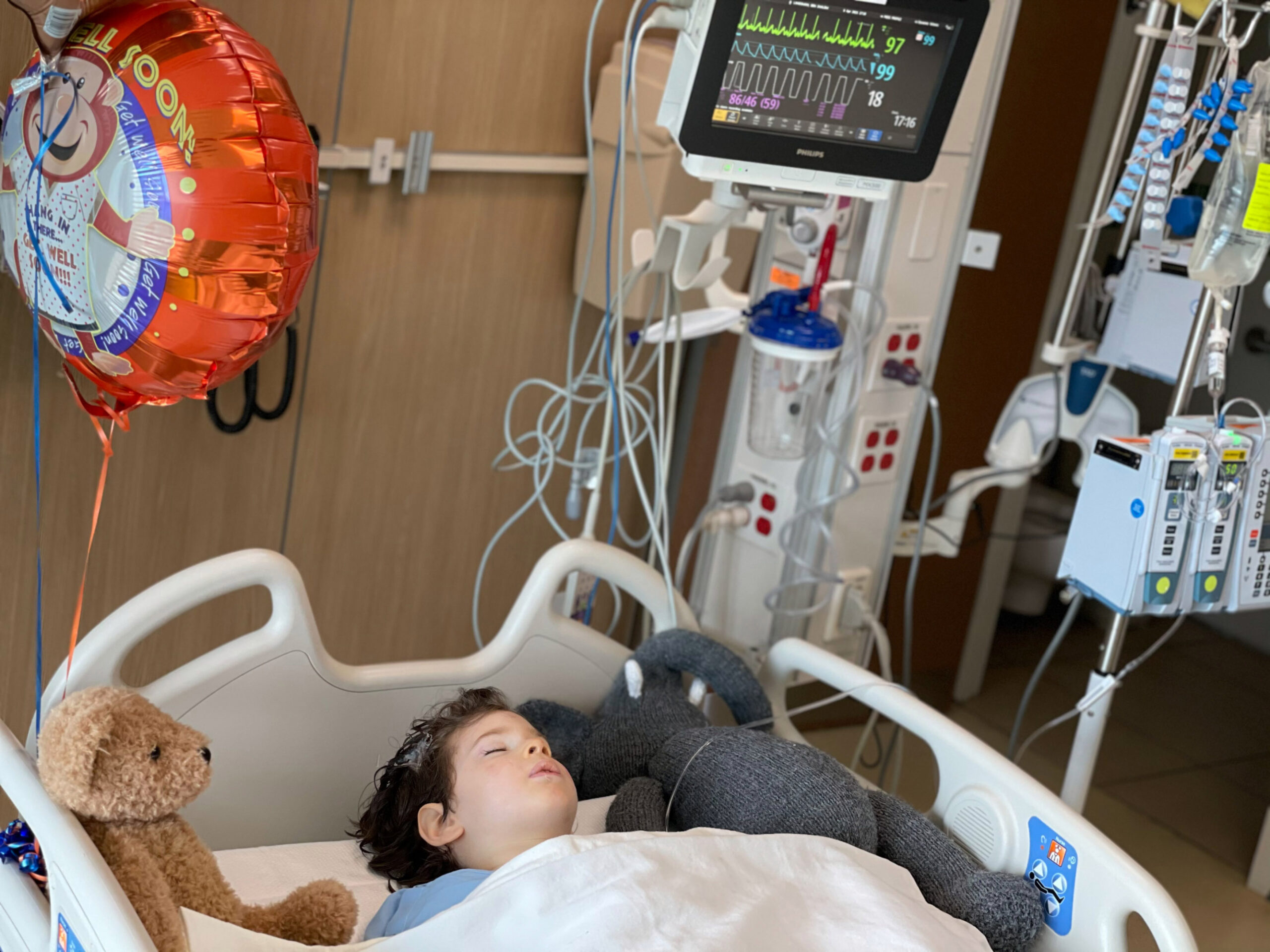
COURTESY OF JENNIE LANDSMAN
The Dayton trial won a greenlight in December and began barely in time for Benny, who will hit the age cutoff of five years in June. “Benny is the pilot. Benny is the ‘God, we hope this works’ kid,” says Rockwell, who doesn’t yet have a date for her daughter’s procedure.
What’s the chance the therapy works? Gene-replacement techniques have been having notable successes, curing kids who don’t have immune systems, and preventing brain diseases. Since 2017, a small number of gene therapies have also been approved for sale in the US, at prices as high as $2.1 million per child.
Record prices have stoked interest among specialist biotech companies, which now see a business even in super-rare diseases. One, called Aspa Therapeutics, says it has plans to initiate a different Canavan gene-therapy trial. Its CEO, Eric David, estimates there are 1,000 children alive with the disease in the US and Europe. “That, for us, is enough,” he says.
There’s no certainty gene therapy will succeed in Canavan. Even if the corrected gene stops the disease from progressing, the kids’ brains may have already been irreversibly damaged.
“I hope she will sit up on her own, maybe say Mommy and Daddy,” says Rockwell of her daughter. “I am hopeful, but it is purely experimental. We are handing our babies over to science and hoping and praying it works.” It will be a month before doctors know if the new gene is functioning in Benny’s brain, but likely much longer to know of any effect on his symptoms.
In a message to donors, Gary Landsman addressed what he called the “loaded” question of what he expects the procedure to achieve.
“I’ve pondered this question over and over and over again,” he wrote. “Is it OK to want more? Is it OK to want to hold their hands as they walk beside me? Is it OK to want to hear them speak to me? Perhaps I am playing a dangerous game with my psyche. But I think the hope it provides is worth the risk.”




