A mouse embryo has been grown in an artificial womb—humans could be next
The photographs alone tell a fantastic story—a mouse embryo, complete with beating heart cells, a head, and the beginning of limbs, alive and growing in a glass jar.
According to a scientific group in Israel, which took the picture, the researchers have grown mice in an artificial womb for as long as 11 or 12 days, about half the animal’s natural gestation period.
It’s record for development of a mammal outside the womb, and according to the research team, human embryos could be next—raising huge new ethical questions.
“This sets the stage for other species,” says Jacob Hanna, a developmental biologist at the Weizmann Institute of Science, who led the research team. “I hope that it will allow scientists to grow human embryos until week five.”
Growing human embryos in the lab for that long, deep into the first trimester, would put science on a collision course with the abortion debate. Hanna believes lab-grown embryos could be a research substitute for tissue derived from abortions, and possibly a source of tissue for medical treatments as well.
How they did it
Hanna’s team grew the mouse embryos longer by adding blood serum from human umbilical cords, agitating them in glass jars, and pumping in a pressurized oxygen mixture. Hanna likens the process to putting a covid-19 patient on a ventilation machine.
“That forces the oxygen into the cells,” he says. “Then the patient is much happier. You can see it has a blood system and all the major organ systems are working.”

The mouse embryos only died after they became too large for the oxygen to diffuse through them, since they lack the natural blood supply a placenta could provide.
The work creates a scientific window onto the early embryo, which is normally hidden inside the uterus. In a publication today in the journal Nature, the Israeli team describes a series of experiments in which they added toxins, dyes, viruses, and human cells to the developing embryonic mice, all to study what would occur.
“It’s a tour de force—very, very impressive,” says Alfonso Martinez Arias, a developmental biologist and stem cell researcher based at Pompeu Fabra University in Spain, who was not involved in the research.
Next: humans
Hanna says scientists will want to develop human embryos this way too. He recognizes that images of lab-grown human embryos with a roughly recognizable shape—head, tail, and limb buds—could be shocking. The human equivalent of Hanna’s 12-day-old mice would be a first-trimester embryo.
“I do understand the difficulties. I understand. You are entering the domain of abortions,” says Hanna. However, he says he can rationalize such experiments because researchers already study five-day-old human embryos from IVF clinics, which are also destroyed in that process.
“So I would advocate growing it until day 40 and then disposing of it,” says Hanna. “Instead of getting tissue from abortions, let’s take a blastocyst and grow it.”
The research is part of an explosion of new techniques and ideas for studying early development. Today, in the same issue of Nature, two other research groups are reporting a leap forward in creating “artificial” human embryos.
Those teams managed to coax ordinary skin cells and stem cells to self-assemble into look-alike early human embryos they call “blastoids,” which they grew for about 10 days in the lab. Several kinds of artificial models of embryos have been described before, but those described today are among the most complete, because they possess the cells needed to form a placenta. That means they are a step closer to being viable human embryos that could develop further, even until birth.
Scientists say that they would never try to establish a pregnancy with artificial embryos—an act that would be forbidden today in most countries.
Instead, Hanna says, an obvious next step would be to add these embryo models to his system of spinning jars and see how much further they can develop. “It took six years of very intense work to get this system to where it is,” says Hanna. “We do have the goal to do it with synthetic embryos as well.”
Early days
For now, the artificial womb technology remains “complex and expensive,” says Martinez Arias. He does not believe many other labs will be able to use it, limiting its impact in the short term, and he is not in favor of growing human embryos this way: “It’s expensive and complicated, so we will have to see how useful it is.”
The mouse-in-a-jar technology needs other improvements, too, Hanna says. He was not able to grow the mice starting from a fertilized egg all the way to day 12. Instead, he collected 5-day-old embryos from pregnant mice and moved them into the incubator system, where they lived another week.
The issue is that currently, the mouse embryos develop correctly only if they can be attached to an actual mouse uterus, at least for a brief time. Hanna’s team is working on adapting the procedure so they can develop the mice entirely in vitro.
Hanna says he’s not interested in bringing mice to term inside the lab. His goal is to watch and manipulate early development. “I want to see how the program unfolds,” he says. “I have plenty to study.”
Banned?
Long-term studies of live human embryos developing in the lab are currently banned under the so-called 14-day rule, a guideline (and a law in some countries) according to which embryologists have been forbidden to grow human embryos more than two weeks.
However, a key scientific organization, the International Society for Stem Cell Research, or ISSCR, has plans to recommend rescinding the prohibition and allowing some embryos to grow for longer.
Hanna says that means he could grow human embryos in his incubator—so long as Israeli ethics boards sign off, something he thinks they would do.
“Once the guidelines are updated, I can apply, and it will be approved. It’s a very important experiment,” says Hanna. “We need to see human embryos gastrulate and form organs and start perturbing it. The benefit of growing human embryos to week three, week four, week five is invaluable. I think those experiments should at least be considered. If we can get to an advanced human embryo, we can learn so much.”
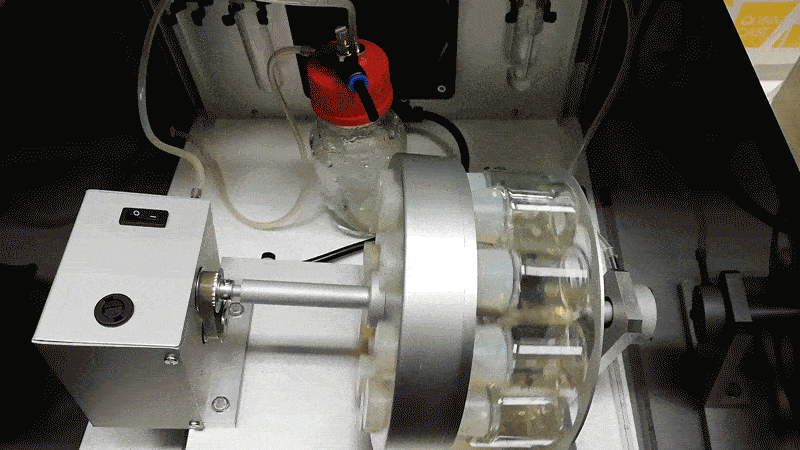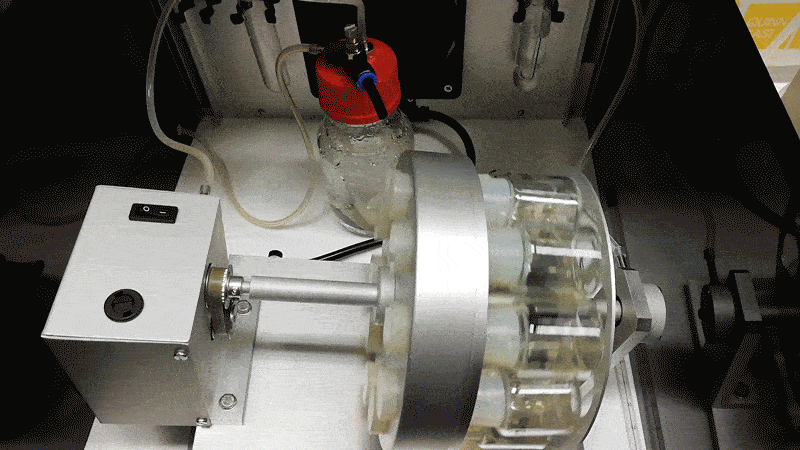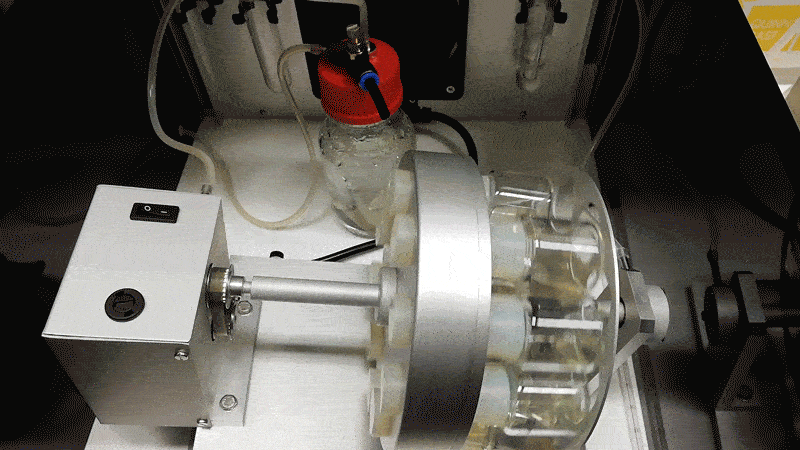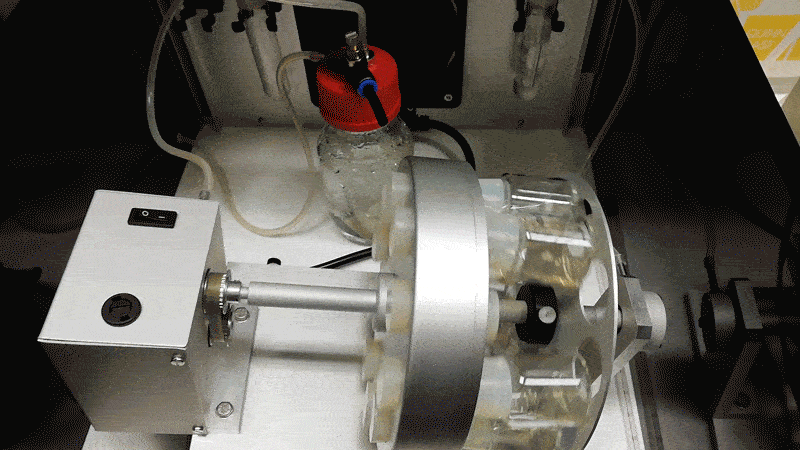
Hanna says to make such experiments more acceptable, human embryos could be altered to limit their potential to develop fully. One possibility would be to install genetic mutations in a calcium channel so as to prevent the heart from ever beating.
I asked Hanna if he had sought the advice of ethicists or religious figures. He said he has not. Instead, he is awaiting the advice of his professional body and ethics clearance from his university.
“The ISSCR is my rabbi,” he says.
There may be unexpected practical applications of growing human embryos in jars. William Hurlbut, a doctor and bioethicist at Stanford University, says the system suggests to him a way to obtain primitive organs, like liver or pancreas cells, from first-trimester human embryos, which could then be grown further and used in transplant medicine. Hanna agrees this is a potential direction for the technology.
“The scientific frontier is moving from molecules and test tubes to living organisms,” says Hurlbut. “I don’t think that organ harvesting is so far-fetched. It could eventually get there. But it’s very fraught, because one person’s boundary is not another person’s boundary.”

